Advancements in regenerative platelet therapy


The Use of Blood Platelets in Regenerative Medicine
Over the last two decades, many attempts have been made in the field of wound regeneration with the aim of predictably repairing, regenerating, or restoring damaged and diseased tissues. These include strategies which utilize foreign materials often derived from allografts, xenografts, or synthetically produced alloplasts to regenerate host tissues [1-4]. Although these materials have shown promise in various aspects of regenerative medicine, it is important to note that all of these methods create a “foreign body reaction”, whereby a foreign material reacts with human host tissue. This concept in wound healing has led to a push in the development of natural autologous treatment methods with the aim of controlling and improving the healing process. This desire to use autologous grafting materials to improve wound healing characteristics has led to many advancements in blood platelet therapy protocols.
The concept of gathering platelets from blood, as a means of regenerating tissue, dates back over 20 years with the advent of PRP technology. This technology was created to utilize human blood proteins as a source of growth factors capable of supporting tissue growth [5]. This area of research was of great interest for many clinicians because blood platelets have been shown to secrete a number of important growth factors that are responsible for controlling the healing process. These platelet components include: platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), coagulation factors, adhesion molecules, cytokines, chemokines, and a variety of other angiogenic factors capable of stimulating the proliferation and activation of cells involved in the wound healing process [6].
This understanding of blood platelet concentrations resulted in the discovery of PRP in the 1990’s [7]. Although PRP was an incredible technology for it’s time, several drawbacks prevent PRP from becoming more widely used today. Due to the centrifugation protocols implemented by the PRP process, the use of anti-coagulants is necessary to prevent clotting. These anti-clotting agents include bovine thrombin or CaCl2, both known to be inhibitors of wound healing [11]. Another drawback of PRP is the lengthy time of harvesting, centrifugation, and processing. This makes the implementation of PRP in small surgeries difficult, as treatment time is an important aspect of effective patient care. The most crucial drawback of PRP is the length of time in which growth factors are released once introduced to a surgical site. Clinical research has shown that the release of growth factors from PRP is much faster than the release rate shown in S-PRF™, A-PRF™ & I-PRF™ [8]. It has been suggested that a preferential release of growth factors may be obtained by a more slowly releasing curve over time, opposed to a quick and short burst, as found using PRP [8-10]. This slow release of growth factors through the entire healing process is essential for proper wound healing as various cells preform different functions during the various stages of healing [18,32]. The desire to create a more optimized method of reintroducing growth factors over a longer period resulted in the creation of PRF: the next generation of platelet wound healing.
Advanced PRF for Regenerative Wound Healing
Platelet Rich Fibrin: Advancements in Platelet Wound Healing
The creation of PRF was a direct response to the drawbacks and limitations of PRP in regenerative medicine. In the early 2000’s the objective of many researchers was to create a method of delivering growth factors without the use of anti-coagulates or other foreign additives [11]. One of the doctors at the forefront of this research was Dr. Joseph Choukroun, a pain management specialist from France. Dr. Choukroun is responsible for conducting countless studies on wound healing and tissue grafting and is credited with the invention of L-PRF, Advanced A-PRF™,Injectable I-PRF+™, and Sticky Bone S-PRF™. Dr. Choukroun initially tested various spin protocols at relatively high speeds without the use of additives. This research resulted in a three-dimensional fibrin matrix, termed L-PRF™ [12-14]. Unlike PRP, the elimination of anti-coagulants enabled clinicians to create PRF as both a fibrin clot as well as acellular plasma. Within the fibrin matrix are varieties of platelet growth factors responsible for controlling the wound healing process [18].
When evaluating the concentrations of growth factors within PRF, it is important to examine their biological roles in regards to wound healing. PRF contains TGF-beta, a known agent responsible for the rapid proliferation of various cell types found in the oral cavity [15, 16]. Another important growth factor is PFGF, an essential regulator for the migration, proliferation, and survival of mesenchymal cells. The third important growth factor in PRF is VEGF, responsible for angiogenesis and future blood flow to damaged tissues [17]. Research has shown that the concentration and release rate of these growth factors plays a tremendous role in wound healing [18]. Knowing this, Dr. Choukroun set out to create optimized centrifugation parameters, which allows for the creation of a highly concentrated fibrin matrix that has a slower rate of growth factor release. After years of research examining the impact of spin speeds and spin time on the structure of PRF, Dr. Choukroun developed new centrifugation parameters using the low-speed centrifugation concept (LSCC). This concept states that, at lower rotational speeds, the concentration of growth factors increases due to a reduction in cell “pull down” [19]. Since the discovery of LSCC, researchers have tested various centrifugation speeds and exposure times and have monitored the changes to cell structure and density. This research into protocol optimization led to the discovery A-PRF™, I-PRF+™, and S-PRF™ which were developed using the LSCC.



Advanced A-PRF™, Injectable I-PRF™ & Sticky Bone S-PRF™
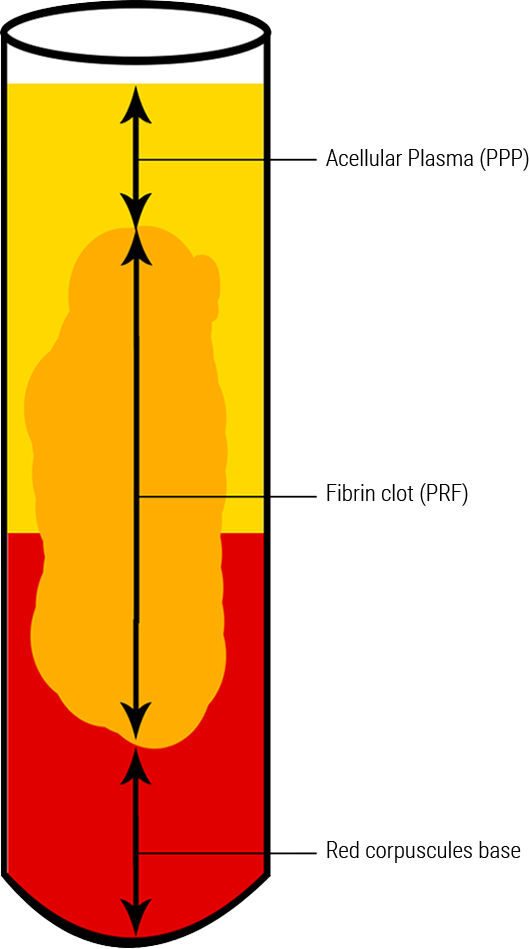
To understand the advantages of A-PRF™, S-PRF™, I-PRF™ it is important to understand what is happening when blood is subjected to the forces of centrifugation. When blood is spun within a centrifuge, a g-force is applied to the collection tubes due to the rotation of the centrifuge rotor. As a result, the blood within the collection tubes is subjected to the same force, driving growth factors to the bottom of the collection tubes. The faster the rotational speed, the larger the force applied to the blood within the tubes. Therefore, a reduction in the centrifugation speed results in a reduction of the force driving cells downwards towards the bottom of the tube (Figure 1.1) [19]. After a fibrin membrane is created using centrifugation, the bottom of the clot that is dense with red blood cells is removed before introducing the clot to a surgical site with LSCC protocols. The migration of growth factors to the bottom of the clot is minimized, resulting in less growth factors being removed during processing [20].
The reduction in growth factor removal during processing produces a highly concentrated PRF clot, which has many benefits when compared to PRP and L-PRF™. New A-PRF™,S-PRF™ & I-PRF+™ have shown a higher total release of growth factors when compared to PRP over a 14-day period. It has also been shown that both A-PRF and A-PRF+ have significantly higher levels of human gingival fibroblast migration and proliferation when compared to L-PRF™ [20]. In a study conducted by Dr. Choukroun and his colleagues, it was also shown that the use of LSCC increased growth factor release of TGF-beta1, PDGF-AA, PFGF-AB, PDGF-BB, VEGF, IGF, and EGF when compared to high-speed protocols [20]. This change in growth factor concentration and release time is what separates A-PRF™,S-PRF™, and I-PRF+™ protocols from older methods of blood centrifugation, such as those implemented by PRP and L-PRF™.
Applications of A-PRF™, I-PRF+™, and S-PRF™
The indications of A-PRF™, I-PRF+™,and S-PRF™ are widespread and include the following applications:
- Extraction Sockets [21]
- Sinus Grafting [25]
- Root Coverage [27]
- Intrabony & Furcation Defects [28]
- Peri-implant Defects [29]
- Around Dental Implants [30]
- GBR [31]
- Facial Injections & Orthopedics [32]
The Process for PRF System
The use of A-PRF™,I-PRF+™, and S-PRF™ pose a variety of advantages that benefit both patient and clinician. Listed below are some of the advantages that the Process for PRF system can provide to your practice:
- Reduction in Surgical Costs — Material costs is approximately $20/patient
- Increased Tissue Growth — Higher growth factor release compared to PRP and L-PRF™ [20]
- Natural Healing — A-PRF™, I-PRF+™, and S-PRF™ are processed entirely without the use of additives [8]
- Safe to Use — All components of the Process for PRF system have been cleared with the FDA
- Infection Resistance — Shown to have as much as a 10-fold decrease in osteomyelitis infections [21]
- Graft Additive — Used to improve the handling characteristics of grafting materials- "Sticky Bone" [25]
- Improves Surgical Success — Uses growth factors to improve the likelihood of surgical success
- Reduces Patient Pain — Has been shown to reduce postoperative pain [22]
- Reduces Healing Time — Ability to shorten the overall healing period [23-26]
A-PRF™ & S-PRF™ Clinical Cases
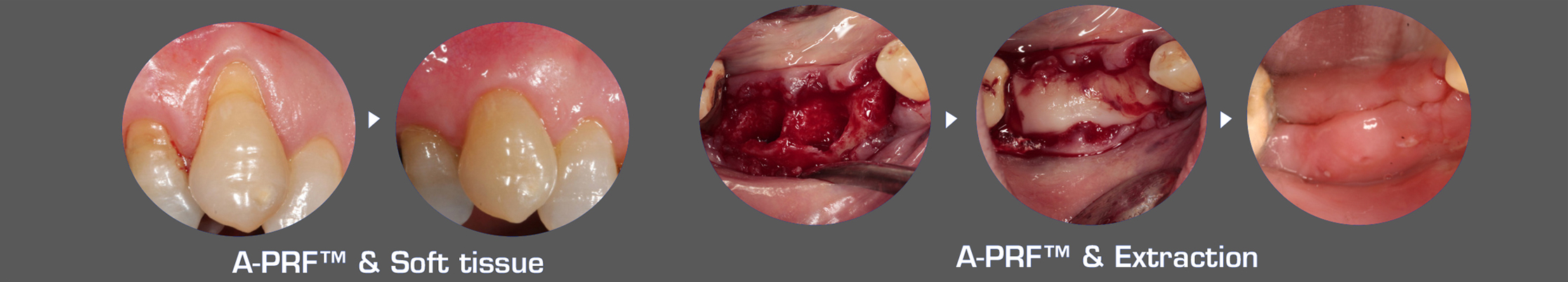
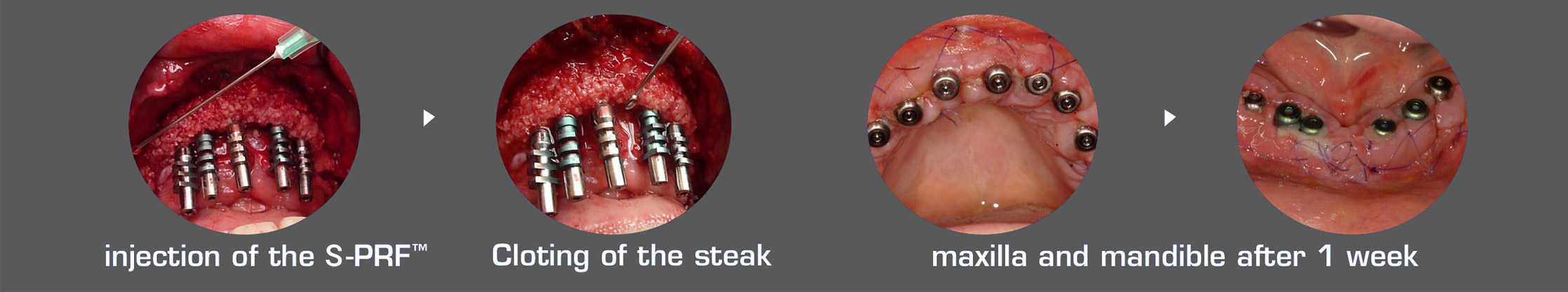
Improving surgical success with A-PRF™, S-PRF™, & I-PRF+™
PRF as a Tool For Clinical Success
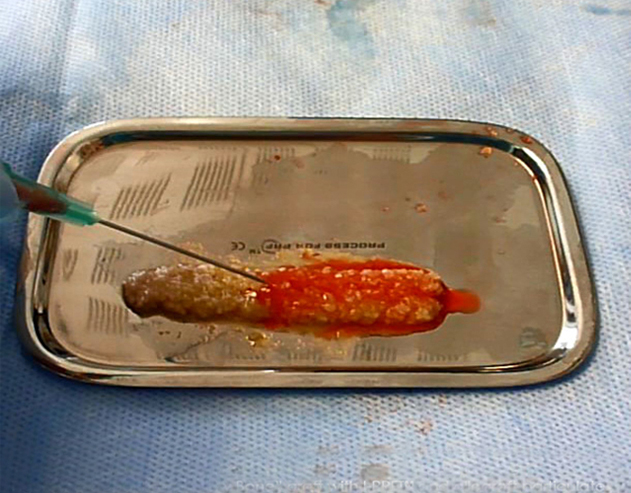
Using A-PRF™, S-PRF™ & I-PRF+™ ensures that you are providing your patients with the highest likelihood of surgical success, especially when the health of the patient is not ideal. During challenging procedures, A-PRF™, S-PRF™ & I-PRF+™ can be used to not only improve surgical results, but also ease the difficulty of performing surgical operations. The combination of grafting materials with A-PRF™ exudate & S-PRF™ yields a material called “sticky bone”; a jelly-like matrix of grafting particulate and solidified acellular plasma (Figure 1.2)[25]. This unique material provides surgeons with optimal graft handling characteristics, making the transportation and application of grafting materials simple and predictable. A-PRF™ fibrin clots can be manipulated using the A-PRF Box MK2. This devise allows for the creation of both fibrin membranes, cylindrical plugs & ponchos that can be used as barrier membranes [22].
The use of I-PRF+™ for facial injections is becoming a popular alternative to current PRP and Botox® treatments for a variety of reasons. I-PRF+™ provides a natural and safe alternative to traditional injection fillers, which allows clinicians to inject the site directly with growth factors to stimulate collagen growth without the fear of toxicity or infection [32]. The injection of I-PRF+™ creates a microenvironment capable of slowly releasing growth factors over an extended period. Leukocytes from the I-PRF™ provoke the physiological inflammation process by secreting an increased concentration of leukocyte growth factors. The advantages of I-PRF+™ are also seen in orthopedics. I-PRF+™ is becoming a popular option with orthopedic surgeons as an alternative to PRP. This is because of the low inflammatory response caused I-PRF+™ and the addition of lukocytes which aids in the healing process. I-PRF+™ can be injected safely into knees and other joints without risk of infection or pain.
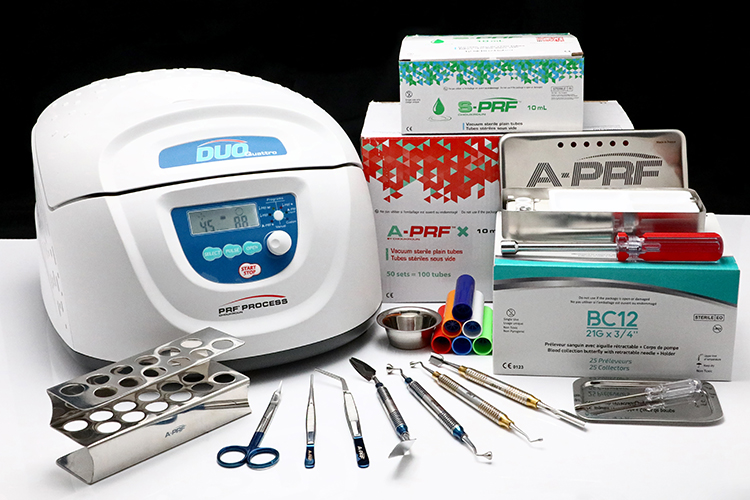
The Process for PRF Systems
Starter System: PRF-STARTERKIT
Includes: Centrifuge, PRF-BCK, PRF-IS (See Below)
Professional System: PRF-PROKIT
Includes: Centrifuge, PRF-BCK, 2x PRF-IS (See Below)
Blood Collection Kit: PRF-BCK
Includes items below
- Reusable tourniquetPRF-TORNIQUET
- Blood collection butterflies 21G (24/box)PRF-PRVT
- Red-top collection tubes (100/box)PRF-A-PRF+
- Green-top collection tubes (24/box)PRF-S-PRF
- PomCol Butterfly cooling systemPRF-PomCol
PRF Instrument Set: PRF-IS
Includes items below
- MK2 A-PRF™ BOXPRF-PRFBOXMK2
- PolySteribox™ sterilization cassettePRF-STERIBOX
- PolySteribox™ silicon bedPRF-BED
- Curved goldman fox PRF scissors, TCPRF-CSX
- PRF forceps straightPRF-FORCEPS
- PRF forceps giraffePRF-GIRAFE
- Double ended graft spoonPRF-D.SPOON
- Large graft packerPRF-BIGCOMPACT
- Small graft packerPRF-SMALLCOMPACT
- PRF padPRF-PAD
- Rectangular PRF trayPRF-TRAY
- PRF bowlPRF-BOWL
- PRF mini trayPRF-MINITRAY
- Test tube rackPRF-TUBEHOLDER
- Membrane trayPRF-MEMTRAY
- PomPac tube cooling systemPRF-PomPac

-

DUO QUATTRO CENTRIFUGE
The DUO Quattro centrifuge is designed with 6 easy to use pre-programmed settings. These settings allow you to select the appropriate spin protocol depending on the needs of your patient. A 7th setting is also provided which allows clinicians to alter spin speeds and cycle time. The design of the DUO Quatto limits vibration which decreases fibrin decay.
-
BLOOD COLLECTION
The selection of blood collection tube material is dependant on the type of PRF that you wish to create. Red top glass tubes are used to create A-PRF™ fibrin clots, whereas the green top plastic vials are used to create S-PRF™ plasma. Both green and red tubes can be used with the blood collection needle set provided. Both tubes are used with the PomPac™ and PomCol™ to achive larger clots and more optimal clotting times. A series of videos on our website outline these procedures (www.dit-usa.com).

-
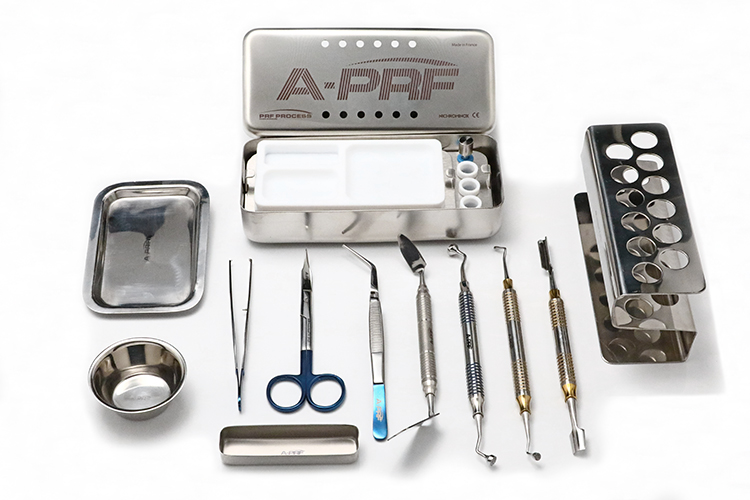
PROCESSING & INSTRUMENTATION
The instruments provided in the Process for PRF system allow for the manipulation and transportation of PRF fibrin clots. Included in the kit is the newly redesigned A-PRF MK2 BOX; a stainless steel container which allows for the pressing of fibrin clots into various forms. The MK2 BOX allows clinicians to produce A-PRF membranes, A-PRF plugs, as well as S-PRF plasma membranes.
Also included in the kit are a series of hand instruments which provide clinicians with the ability to easily modify and transport fibrin membranes. Stainless steel bowls and a tray are included in the kit which gives clinicians a sterile working surface to modify fibrin clots and mix grafting materials. These instruments provide clinicians with all of the tooling needed to process both A-PRF™ and S-PRF™ easily and predictably.
-
STERILIZATION & INSTRUMENT CARE
The instruments provided by the Process for PRF system can be easily transported and sterilized in the PolySteribox. This cassette has a unique PTFE filter which allows the box to be sterilized outside of a sterile pack. Once sterilized, the Polysteribox filters will keep the contents inside the box sterile for several weeks. Due to its semi-transparent material, the contents of the PolySteribox are visible and can be examined without opening the box. Sterilization seals indicate if the box has already been sterilized. Maintenance of the Polysteribox is simple, with only basic visual checks needed. This cassette allows you to both store and sterilize all of the instrumentation provided in the Process for PRF system.

References
- Coury AJ. Expediting the transition from replacement medicine to tissue engineering. Regenerative biomaterials. 2016;3(2):111-3
- Dai R, Wang Z, Samanipour R, Koo KI, Kim K. Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Stem cells international. 2016;2016:6737345
- Rouwkema J, Khademhosseini A. Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol.2016.
- Zhu W, MA X, Gou M, Mei D, Zhang K, Chen S. 3D printing of functional biomaterials for tissue engineering. Current opinion in biotechnology.2016;40:103-12
- Upputuri PK, Sivasubramanian K, Mark CS, Pramanik M. Recent developments in vascular imaging techniques in tissue engineering and regenerative medicine. BioMed research international. 2015;2015:783983
- Nurden AT. Platelets, inflammation and tissue regeneration. Annals of Oral & Maxillofacial Surgery. 2013;1(1):10.
- de Vries RA, de Bruin M, Marx JJ, Mart HC, Van de Wiel A. Viability of platelets collected by apheresis versus the platelet-rich plasma technique: a direct comparison. Transfusion science. 1993;14(4):391-8
- Kobayashi E, Fluckiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clinical oral investigations. 2016.
- Lucarelli E, Beretta R, Dozza B, Tazzari PL, O’Connel SM, Ricci F, et al. A recently developed bifacial platelet-rich fibrin matrix. European cells & materials. 2010;20;13-23
- Saluja H, Dehane V, Mahindra U. Platelet Rich fibrin: a second generation platelet concentrate and new friend of oral and maxillofacial surgeons. Annals of maxillofacial surgery. 2011;1(1):53-7
- Choujroun J, Adda F, Schoeffler C, Vervelle A. Une Opportunite en paro-implantologie: le PRF. Implantodontie. 2001;42(55):e62.
- Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, et al. Platelet-rich fibrin(PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral-Surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2006;101(3):e56-60
- Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral surgery, oral medicine, oral pathology oral radiology, and endodontics: 2006;101(3):e37-44.
- Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): a second generation platelet concentrate. Part II: platelet-related biological features. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2006:101(3):e34-50
- Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. The New England journal of medicine. 1994;331(19):1286-92
- Bowen T, Jenkins RH, Fraser DJ. MicroRNAs, transforming growth factor beta-1, and tissue fibrosis, The journal of pathology. 2013;229(2):274-85
- Shamloo A, Xu H, Heilshorn S. Mechanisms of vascular endothelial growth factor-induced pathfinding by endothelial sprouts in biomaterials. Tissue engineering Part A. 2012;18(3-4):320-30
- Eren G, Gurkan A, Atmaca H, Donmez A, Atilla G. Effect of centrifugation time of growth factor and MMP release of an experimental platelet-rich fibrin-type product. Platelets. 2016;27(5):427-32
- El Bagdadi K, Yu X, Al-Maawi S, Dias A, Dohle E, Kubesch A, Booms P, Sader R, Kirkpatrick J, Choukroun J, Ghanaati S. Reduction of relative centrifugal forces influences the growth factor release within the solid PRF-based matrices: A prood of concept of LSCC ( Low Speed Sentrifugation Concept, ETOJ. In revision, (2016).
- Fujiooka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang y, Choukroun J. Optimized Platelet Rich Fibrin With the Lowe Speed Concept: Growth Factor Release, Biocompatibility and Cellular Response. J Periodontal 2017;88(1):112-121. Epub 2016 Sep 2.
- Hoaglin DR, Lines GK, Prevention of localized osteitis in mandibular third-molar sites using platelet-rich fibrin. International journal of dentistry. 2013;2013:875380
- Bilginaylar K, Uyanik LO. Evaluation of the effects of platelet-rich fibrin and piezosurgery on outcomes after removal of impacted mandibular third molars. The British journal of oral & maxillofacial surgery. 2016;54(6):629-33
- Inchingolo F, Tatullo M, Marrilli M, et al. Trial with Platelet-Rich Fibrin and Bio-Oss used as grafting materials in the treatment of the severe maxillar bone atrophy: clinical and radiological evaluations. European review for medical and pharmacological sciences. 2010;14(12):1075-84
- Tatullo M, Marrelli M, Cassetta M, Pacifici A, Stefanelli LV, Scacco S, et al. Platelet Rich Fibrin (P.R.F) in reconstructive surgery of atrophied maxillary bones: clinical and histological evaluations. International journal of medical sciences. 2012;9(10):872-80
- Zhang Y, Tangl S, Huber CD, Lin Y, Qiu L, Rausch-Fan X, Effects of Choukroun’s platelet-rich fibrin on bone rengeration in combination with deprotonized bovine bone mineral in maxillary sinus augmentation: a histological and histomorphometric study. Journal of cranio-maxillo facial surgery: official publication of the European Association for Craino-Maxillo-Facial Surgery. 2012;40(4):321-8
- Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2006;101(3):299-303
- Agarwal SK, Jhingran R, Baines VK, Srivastava R, Maden R, Rizvi I. Patient-centered evaluation of microsurgical management of gingival recession using coronally advanced flap with platelet-rich fibrin or amnion membrane: a comparative analysis. European journal of dentistry. 2016;10(1):121-33
- Agarwal A, Gupta ND, Jain A. Platelet rich fibrin combined with decalcified freeze-dried bone allograft for the treatment of human intrabony periodontal defects: a randomized split mouth clinical trail. Acta odontologica Scandinavica. 2016;74(1):36-43
- Hao P-J, Wang Z-G, XU Q-c, Xu S, Li Z-R, Yang P-S, et al. Effect of umbilical cord mesenchymal stem cells in peri-implant bone defects after immediate implant; an experiment study in beagle dogs. International journal of clinical and experimental pathology. 2014;7(11):8271.
- Miron RJ, Fujioka-Kobayashi M, Bishara M, Zhang Y, Hernadez M, Choukroun J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Engineering Part B: Reviews. 2016.
- Miron RJ, Fujioka-Kobayashi M, Bishara M, Zhang Y, Hernandez M, Choukroun J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A systematic Review. Tissue engineering Part B, Reviews.2016.
- Kim DH, Je YJ, Kim CD, Lee YH, Seo YJ, Lee JH, et al. Can Platelet-rich fibrin be used for skin rejuvenation? Evaluation of effects of platelet-rich fibrin on human dermal fibroblasts. Annals of dermatology. 20122;23(4):424-31
- Tan KW, Chong SZ, Wong FHS, Evard M, Tan SM-L, Keeble J, Kemeny DM, Ng LG, Abastado J-P, Angeli V. Neutrophils contribute to inflammatory lymphangiogenesis by increasing VEGF-D., Blood. 2013;122:3666-77
L-PRF™ is a registered trademark of Intra-Lock®International Inc.
Dental Implant Technologies®
Global Headquarters
9414 E. San Salvador Dr, #112,Scottsdale, AZ 85258
Telephone: (800) 452-0582
www.dit-usa.com
PRF Catalogs





